🅰I
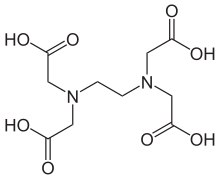
The most important process for the elimination of EDTA from surface waters is direct photolysis at wavelengths below 400 nm.[31] Depending on the light conditions, the photolysis half-lives of iron(III) EDTA in surface waters can range as low as 11.3 minutes up to more than 100 hours.[32] Degradation of FeEDTA, but not EDTA itself, produces iron complexes of the triacetate (ED3A), diacetate (EDDA), and monoacetate (EDMA) – 92% of EDDA and EDMA biodegrades in 20 hours while ED3A displays significantly higher resistance. Many environmentally-abundant EDTA species (such as Mg2+ and Ca2+) are more persistent.